|
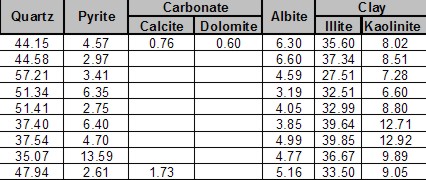
Shale is a fine-grained, clastic sedimentary rock composed of mud that is a mix of clay minerals and tiny fragments (silt-sized particles) of other minerals, especially quartz, dolomite, and calcite. The ratio of clay to other minerals varies.
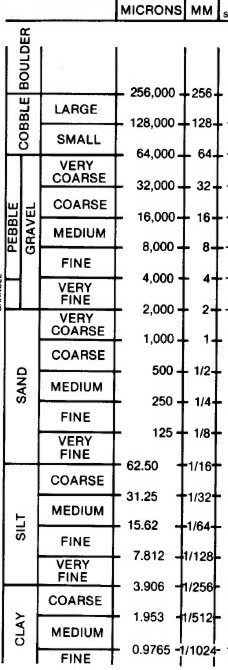
Shale is characterized by breaks along thin laminations, parallel to the bedding. Mudstones are similar in composition but do not usually show layering within the zone. Geologists define clay as any mineral in a rock with a grain size less than 4 microns, even though the mineral may not be a clay mineral. Silt is defined as a rock with particle size between 4 and 62 microns. Silt sized particles are usually non-clay minerals and clay sized particles are usually clay minerals, although non-clay minerals may also fall into this category. From a petrophysical analysis point of view, clay-rich shales have traditionally been called “shales” and non-clay shales have been called “silts”. Petrophysical analysis deals with minerals, not particle size, so it is confusing to us when a zone is called a shale when the logs show little clay is present.
An example is the Montney shale in northeast British Columbia. Ir is roughly 45% quartz, 45% dolomite, 10% other minerals (few of them are clay). The zone is radioactive due to uranium (not due to clay), so it looks a lot like shale on quick look log analysis; density neutron separation and PE values are also close to shale values. This kind of reservoir needs to be treated as a tight sand.
Other so-called "shales", such as the Monterey Shale, the Niobrara, and Milk River, are laminated shaly sands. These sands need to be analyzed with a Laminated Shaly Sand Model, not a Shaly Sand Model. The sand laminations have good porosity and permeability. The shale laminations contain very little.
Others are radioactive silts with clay and kerogen, such as the Haynesville Shale, which is 50% clay and 50% quartz and calcite. This shale has low effective porosity and very poor permeability. Total organic content is moderately high and there is adsorbed gas, so it gets treated as a true gas shale.
Using the wrong log analysis model, or the wrong assumption as to the character of the zone, will produce silly results, so be sure to understand what type of "gas shale" you are dealing with. Natural fractures in gas shales are an important component in assessing productivity. Fracture analysis using formation resistivity images and acoustic televiewer images is covered elsewhere in this Handbook. Below is a series of core photos of a gas shale showing the laminated nature of shale. Gas is adsorbed in the microporosity on the clay surfaces. The natural fractures along the shale partings help move gas to the well bore when well bore pressure is below formation pressure.
Clay minerals are hydrous aluminum silicates, with variable amounts of iron, magnesium, alkali metals, alkaline earths, and other cations. Clays have structures similar to mica and form flat hexagonal sheets. Clay minerals are common weathering products of feldspar and low temperature hydrothermal alteration of granite. Clay minerals are very common in fine grained sedimentary rocks such as shale, mudstone and siltstone and in fine grained metamorphic slate. Clay minerals include the following groups:
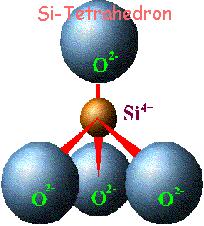
Clay minerals are characterized by two-dimensional sheets of corner sharing SiO4 and AlO4 tetrahedra. These tetrahedral sheets have the chemical composition (Al,Si)3O4, and each tetrahedron shares 3 of its vertex oxygen atoms with other tetrahedra, forming a hexagonal array in two-dimensions. The fourth vertex is not shared with another tetrahedron and all of the tetrahedra point in the same direction, that is, all of the unshared vertices are on the same side of the sheet).
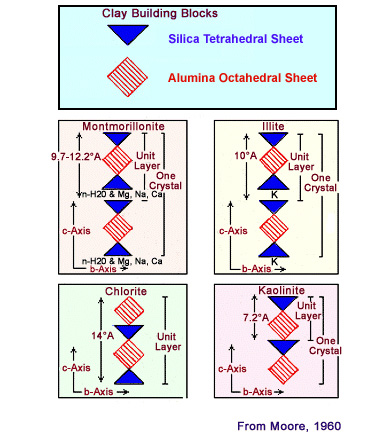
Depending on the
composition of the tetrahedral and octahedral
sheets, the layer will have no charge, or will have
a net negative charge. If the layers are charged,
this charge is balanced by interlayer cations such
as Na+ or K+. Unit layer
arrangement for the four common clay minerals: montmorillonite,
illite, chlorite, and kaolinite. The water in the interlayer of
mont-morillomite makes this a "serlling clay". When montmorillonite
(smectite) clay imbibes fresh water, it swells to several times its
original (dry) volume and retains a good deal of water between
layers in its mineral structure. This change in volume can cause
montmorillonite clays to dislodge and migrate within the pore
system, thus resulting in plugged pore throats. Cation exchange capacity (CEC) is the capacity of a material, such as clay or soil, for ion exchange of positively charged ions between the clay and the surrounding water. A positively-charged ion, which has fewer electrons than protons, is known as a cation. The quantity of positively charged ions (cations) that a clay mineral can accommodate on its negatively charged surface is expressed in milli-equivalent per 100 g, or meq per 100 g. Clays are aluminosilicates in which some of the aluminium and silicon ions have been replaced by elements with different valence, or charge. For example, aluminium may be replaced by iron or magnesium, leading to a net negative charge. This charge attracts cations when the clay is immersed in an electrolyte such as salty water and causes an electrical double layer. The cation-exchange capacity is often expressed in terms of its contribution per unit pore volume, Qv.
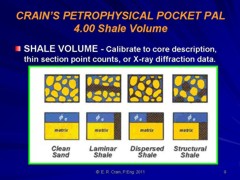
Shale corrections are applied to porosity logs to determine effective porosity, as shown in the illustration above. Since shale contains some water, this water must be subtracted from the total porosity as measured by conventional logging tools. The mathematical method for finding shale volume is the same for all the shale distribution types, but the method for applying the shale correction to the porosity varies.
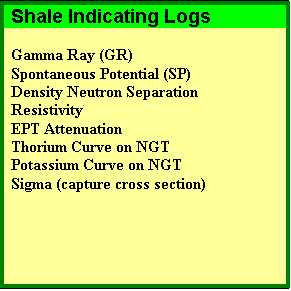
Dispersed shale is usually composed of from clay minerals that form in place after deposition due to chemical reactions between the rock minerals and the chemicals in the formation water. Structural shale is usually deposited as particles, grains, or clasts during the initial depositional phase. For example, the flooding of a river valley can carry mud or shale from surrounding areas. Shale beds range from 100% clay to less than 50%, the balance being other minerals laid down at the same time as the mud. These latter are called limy shales, dolomitic shales, or silty shales. The two most common shale indicating logs are the gamma ray (GR) and spontaneous potential (SP) logs. The units of measurement for GR are API units or counts per second, and for SP are millivolts. The
resistivity, neutron, and sonic are sometimes used individually,
and the separation between density porosity and neutron
porosity is also widely used. More rarely, the electromagnetic
propagation attenuation curve is available and is an excellent
shale indicator, especially in thin bedded (laminated) sand-shale
sequences. |
||
|
Page Views ---- Since 01 Jan 2015
Copyright 2023 by Accessible Petrophysics Ltd. CPH Logo, "CPH", "CPH Gold Member", "CPH Platinum Member", "Crain's Rules", "Meta/Log", "Computer-Ready-Math", "Petro/Fusion Scripts" are Trademarks of the Author |
||


|
||
| Site Navigation | GEOSCIENCE BASICS CLAY and SHALE | Quick Links |



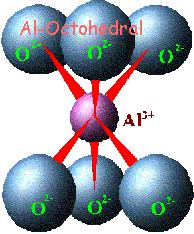

 In
clays, the tetrahedral sheets are always bonded to
octahedral sheets formed from small cations, such as
aluminum or magnesium, coordinated by six oxygen
atoms. The unshared vertex from the tetrahedral
sheet also form part of one side of the octahedral
sheet, but an additional oxygen atom is located
above the gap in the tetrahedral sheet at the center
of the six tetrahedra. This oxygen atom is bonded to
a hydrogen atom forming an OH group in the clay
structure. Clays can be categorized depending on the
way that tetrahedral and octahedral sheets are
packaged into layers. If there is only one
tetrahedral and one octahedral group in each layer
the clay is known as a 1:1 clay. The alternative,
known as a 2:1 clay, has two tetrahedral sheets with
the unshared vertex of each sheet pointing towards
each other and forming each side of the octahedral
sheet.
In
clays, the tetrahedral sheets are always bonded to
octahedral sheets formed from small cations, such as
aluminum or magnesium, coordinated by six oxygen
atoms. The unshared vertex from the tetrahedral
sheet also form part of one side of the octahedral
sheet, but an additional oxygen atom is located
above the gap in the tetrahedral sheet at the center
of the six tetrahedra. This oxygen atom is bonded to
a hydrogen atom forming an OH group in the clay
structure. Clays can be categorized depending on the
way that tetrahedral and octahedral sheets are
packaged into layers. If there is only one
tetrahedral and one octahedral group in each layer
the clay is known as a 1:1 clay. The alternative,
known as a 2:1 clay, has two tetrahedral sheets with
the unshared vertex of each sheet pointing towards
each other and forming each side of the octahedral
sheet.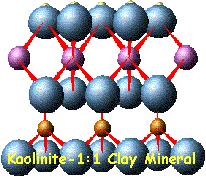
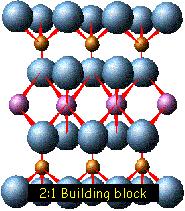
 The crystal
structure of a clay is formed from a stack of layers
interspaced with the interlayers, as shown in the
illustration at the right.
The crystal
structure of a clay is formed from a stack of layers
interspaced with the interlayers, as shown in the
illustration at the right.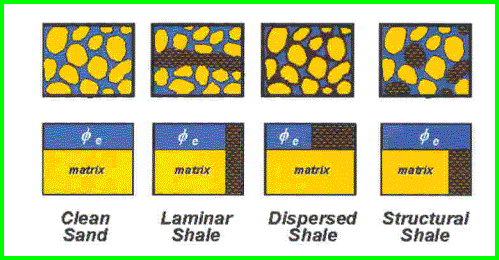
 Laminated
shale is a special case in petrophysical analysis. Standard
models for porosity and saturation do not work - click
Laminated
shale is a special case in petrophysical analysis. Standard
models for porosity and saturation do not work - click