|
 MINERAL
PROPERTIES TABLES MINERAL
PROPERTIES TABLES
Many log analysis models
require prior knowledge of pure mineral properties. There are a
number of sources in service company chartbooks. Several such tables
are shown below as well as a spreadsheet that can be personalized if
you have additional minerals or alternate values.
 CRAIN'S DEFAULT
MATRIX ROCK PROPERTIES
CRAIN'S DEFAULT
MATRIX ROCK PROPERTIES
This spreadsheet gives Crain's default
mineral property values for
the most common sedimentary minerals. The table includes matrix
values for neutron, density, sonic, and photo electric effect used
in 2-mineral models, as well as computed properties based on these
values. These include Uma, Mlith, Nlith, Alith, Klith, and Plith
used in 3-mineral models. K2O content is included in the evaporite
section. This spreadsheet can adjust parameters based on mud
filtrate dalinity.
Download this spreadsheet:
SPR-28 META/LOG MINEROLOGY CALCULATOR
Calculate mineral properties for 2- and 3-mineral
models for different water salinity.
Igneous rocks are covered separately
at the bottom of this page.
 PROPERTIES OF MINERALS
- Version 1
PROPERTIES OF MINERALS
- Version 1
This table lists
the minerals in groups and includes electron density, but not
specific gravity.
|
Name
|
Formula
|
ρlog
g/cc |
φsnp
p.u. |
φcnl
p.u. |
Δtc
μs/ft |
Δts
μs/ft |
Pe
barn/elect |
U barn/cc
|
ε farads/m
|
tp
nsec/m |
GR APR units
|
Σ c.u.
|
|
SILICATES
|
| Quartz
|
SiO2 |
2.64
|
1.
|
2.
|
56.0
|
88.0
|
1.81
|
4.79
|
4.65
|
7.2
|
|
4.26
|
| β
-Cristobalite |
SiO2 |
2.15
|
2.
|
3.
|
|
|
1.81
|
3.89
|
|
|
|
3.52
|
| Opal
(3.5% H2 O) |
SiO2
(H2 O)1209 |
2.13
|
4.
|
2.
|
58.
|
|
1.75
|
3.72
|
|
|
|
5.03
|
| Garnet
|
Fe3
AI2 (SiO4 )3 |
4.31
|
3.
|
7.
|
|
|
11.09
|
47.80
|
|
|
|
44.91
|
|
Hornblende |
Ca2
NaMg2 Fe2 AISi8
O22 (O,OH)2 |
3.20
|
4.
|
8.
|
43.8
|
81.5
|
5.99
|
19.17
|
|
|
|
18.12
|
|
Tourmaline |
NaMg3
AI6 B3 Si6
O2 (OH)4 |
3.02
|
16.
|
22.
|
|
|
2.14
|
6.46
|
|
|
|
7449.82
|
| Zircon
|
ZrSiO4 |
4.50
|
1.
|
3.
|
|
|
69.10
|
311.
|
|
|
|
692
|
|
CARBONATES
|
| Calcite
|
CaCO3 |
2.71
|
0
|
1.
|
49.0
|
88.4
|
5.08
|
13.77
|
7.5
|
9.1
|
|
7.08
|
|
Dolomite |
CaCO3
MgCO3 |
2.85
|
2.
|
1.
|
44.0
|
72.
|
3.14
|
9.00
|
6.8
|
8.7
|
|
4.70
|
| Ankerite
|
Ca(Mg,Fe)(CO3
)2 |
2.86
|
0
|
1.
|
|
|
9.32
|
26.65
|
|
|
|
22.18
|
| Siderite
|
FeCO3 |
3.89
|
5.
|
12.
|
47
|
|
14.69
|
57.14
|
6.8 7.5
|
88 91
|
|
52.31
|
|
OXIDATES
|
| Hematite
|
Fe2
O3 |
5.18
|
4.
|
11.
|
42.9
|
79.3
|
21.48
|
111.27
|
|
|
|
101.37
|
| Magnetite
|
Fe3
O4 |
5.08
|
3.
|
9.
|
73.
|
|
22.24
|
112.98
|
|
|
|
103 08
|
| Geothite
|
FeO(OH)
|
4.34
|
50 +
|
60 +
|
|
|
19.02
|
82.55
|
|
|
|
85 37
|
| Limonite
|
FeO(OH)(H2
O)2 05 |
3.59
|
50 +
|
60 +
|
56.9
|
102.6
|
13.00
|
46.67
|
9.910.9
|
10.511.0
|
|
71.12
|
| Gibbsite
|
AI(OH)3 |
2.49
|
50 +
|
60 +
|
|
|
1.10
|
|
|
|
|
23.11
|
|
PHOSPHATES
|
|
Hydroxyapatite |
Ca5
(PO4 )3
OH
|
3.17
|
5.
|
8.
|
42.
|
|
5.81
|
18.4
|
|
|
|
9.60
|
|
Chlorapatite |
Ca5
(PO4 )3 CI
|
3.18
|
1.
|
1.
|
42.
|
|
606
|
19.27
|
|
|
|
130.21
|
|
Fluorapatite |
Ca5
(PO4 )3 F
|
3.21
|
1.
|
2.
|
42.
|
|
5.82
|
18.68
|
|
|
|
8.48
|
|
Carbonapatite |
(Ca5
(PO4 )3 )2
CO3 H2 O
|
3.13
|
5.
|
8.
|
|
|
5.58
|
17.47
|
|
|
|
9.09
|
|
FELDSPARS-Alkali |
|
Orthoclase |
KAISi3
O8 |
2.52
|
2.
|
3.
|
69.
|
|
2.86
|
7.21
|
4.46.0
|
7.08.2
|
~220
|
15.51
|
|
Anorlhoclase |
KAISi3
O8 |
2.59
|
2.
|
2.
|
|
|
286
|
7.41
|
4.46.0
|
7.08.2
|
~220
|
15.91
|
|
Microciine |
KAISi3
O8 |
2.53
|
2.
|
3.
|
|
|
2.86
|
7.24
|
4.46.0
|
7.08.2
|
~220
|
15.58
|
|
FELDSPARS-Plagioclase |
| Albite
|
NaAISi3
O8 |
2.59
|
1.
|
2.
|
49.
|
85.
|
1.68
|
4.35
|
4.46.0
|
7.08.2
|
|
7.47
|
| Anorthite
|
CaAl2
Si2 O8 |
2.74
|
1.
|
2.
|
45.
|
|
3.13
|
8.58
|
4.46.0
|
7.08.2
|
|
7.24
|
|
MICAS
|
| Muscovite
|
KAl2
(Si3 AIO10 )(OH)2
|
2.82
|
12.
|
20.
|
49.
|
149.
|
2.40
|
6.74
|
6.27.9
|
8.39.4
|
~270
|
16.85
|
|
Glauconite |
K2
(Mg,Fe)2 Al6 (Si4O10)3(OH)2 |
~2.54
|
~23.
|
~38.
|
|
|
6.37
|
16.24
|
|
|
|
24.79
|
| Biotite
|
K(Mg,Fe)3
(AISi3 O10 (OH)2 |
~2.99
|
~11.
|
~21.
|
50.8
|
224.
|
6.27
|
18.75
|
4.86.0
|
7.28.1
|
~275
|
29.83
|
|
Phlogopite |
KMg3
(AISi3 O10 (OH)2 |
|
|
|
50.
|
207.
|
|
|
|
|
|
33.3
|
|
CLAYS
|
| Kaolinite
|
AI4
Si4 O10 (OH)8 |
2.41
|
34.
|
37.
|
|
|
1.83
|
4.44
|
~5.8
|
~8.0
|
80130
|
14.12
|
| Chlorite
|
(Mg,Fe,AI)6 (Si,AI)4 O10
(OH)8 |
2.76
|
37.
|
52.
|
|
|
6.30
|
17.38
|
~5.8
|
~8.0
|
180250
|
24.87
|
| llite
|
K1.15
Al4 (Si76.5 AI11.5
)O20 (OH)4 |
2.52
|
20.
|
30.
|
|
|
3.45
|
8.73
|
~5.8
|
~8.0
|
250300
|
17.58
|
|
Montmorillonite |
(Ca,Na),(AI,Mg,Fe)4
(Si, AI)8 O20 (OH)4
(H2 O)n |
2.12
|
40.
|
44.
|
|
|
2.04
|
4.04
|
~5.8
|
~8.0
|
150200
|
14.12
|
|
EVAPORITES |
|
Halite |
NaCI
|
2.04
|
2.
|
3.
|
67.0
|
120.
|
4.65
|
9.45
|
5.6 6.3
|
7.98.4
|
|
754.2
|
|
Anhydrite |
CaSO4 |
2.98
|
1.
|
2.
|
50.
|
|
5.05
|
14.93
|
6.3
|
8.4
|
|
12.45
|
|
Gypsum |
CaSO4
(H2 O)2 |
2.35
|
50 +
|
60 +
|
52.
|
|
3.99
|
9.37
|
4.1
|
6.8
|
|
18.5
|
| Trona
|
Na2
CO4 NaHCO3 H2
O |
2.08
|
24.
|
35.
|
65.
|
|
0.71
|
1.48
|
|
|
|
15.92
|
|
Tachydrite |
CaCl2
(MgCI2 )2 (H2
O)t2 |
1.66
|
50 +
|
60 +
|
92.
|
|
3.84
|
6.37
|
|
|
|
406.02
|
| Sylvite
|
KCI
|
1.86
|
2.
|
3.
|
|
|
8.51
|
15.83
|
4.64.8
|
7.27.3
|
500 +
|
564.57
|
| Carnalite
|
KCIMgCI2
(H2 O)6 |
1.57
|
41.
|
60 +
|
|
|
4.09
|
6.42
|
|
|
~220
|
368.99
|
|
Langbenite |
K2
SO4 (MgSO4 )2 |
2.82
|
1.
|
2.
|
|
|
3.56
|
10.04
|
|
|
~290
|
24.19
|
|
Polyhalite |
K2
SO4 MgSO4 (CaS04
)2 (H2 O)2 |
2.79
|
14.
|
25.
|
|
|
4.32
|
12.05
|
|
|
~200
|
23.70
|
| Kainite
|
MgSO4
KCI(H2 O)4 |
2.12
|
40.
|
60 +
|
|
|
3.50
|
7.42
|
|
|
~245
|
195.14
|
| Kieserite
|
MgSO4
H2 O |
2.59
|
38
|
43.
|
|
|
1.83
|
4.74
|
|
|
|
13.96
|
| Epsomite
|
MgSO4
(H2 O)2 |
1.71
|
50 +
|
60 +
|
|
|
1.15
|
1.97
|
|
|
|
21.48
|
|
Bischofite |
MgC12
(H2 O)6 |
1.54
|
50 +
|
60 +
|
100.
|
|
2.59
|
3.99
|
|
|
|
323.44
|
| Barite
|
BaSO4 |
4.09
|
1.
|
2.
|
|
|
266 82
|
1091.
|
|
|
|
6.77
|
| Celestite
|
SrSO4 |
3.79
|
1.
|
1.
|
|
|
55.19
|
209.
|
|
|
|
7.90
|
|
SULFIDES
|
| Pyrite
|
FeS2 |
4.99
|
2.
|
3.
|
39.2
|
62.1
|
16.97
|
84.68
|
|
|
|
9010
|
| Marcasite
|
FeS2 |
4.87
|
2
|
3.
|
|
|
16.97
|
82.64
|
|
|
|
88.12
|
|
Pyrrhotite |
Fe7
SB |
4.53
|
2.
|
3.
|
|
|
20.55
|
93.09
|
|
|
|
94.18
|
|
Sphalerite |
ZnS
|
3.85
|
3.
|
3.
|
|
|
35.93
|
138.33
|
7.88.1
|
9.39.5
|
|
25.34
|
|
Chalopyrite |
CuFeS2 |
4.07
|
2.
|
3.
|
|
|
26.72
|
108.75
|
|
|
|
102.13
|
| Galena
|
PbS
|
6.39
|
3.
|
3.
|
|
|
1631.37
|
10424.
|
|
|
|
13.36
|
| Sulfur
|
S
|
2.02
|
2.
|
3.
|
122.
|
|
5.43
|
10.97
|
|
|
|
20.22
|
|
COALS
|
|
Anthracite |
CH358
N009
KO022 |
1.47
|
37.
|
38.
|
105.
|
|
0.16
|
0.23
|
|
|
|
8.65
|
|
Bituminous |
CH793
N015 O078 |
1.24
|
50 +
|
60 +
|
120.
|
|
0.17
|
0.21
|
|
|
|
14.30
|
| Lignite
|
CH849
N015 O211 |
1.19
|
47.
|
52.
|
160.
|
|
0.20
|
0.24
|
|
|
|
12.79
|
 PROPERTIES OF MINERALS
- Version 2 PROPERTIES OF MINERALS
- Version 2
This table lists
the minerals in groups and includes both specific gravity and
electron density but excludes compressional and shear sonic values.
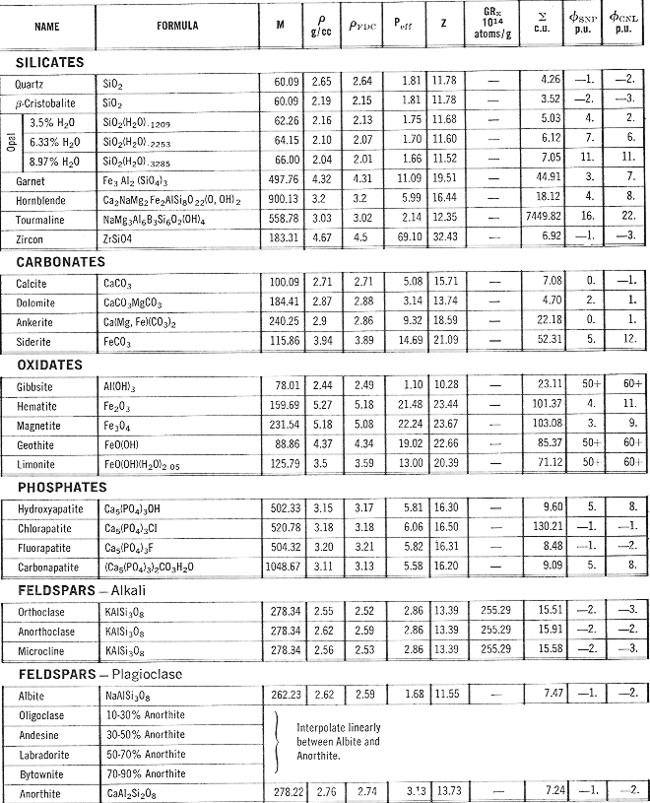
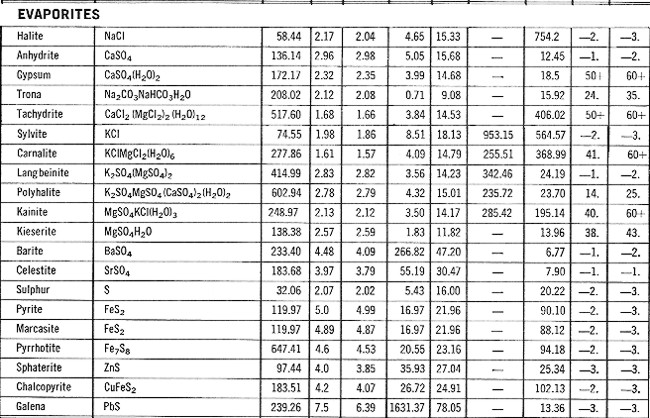
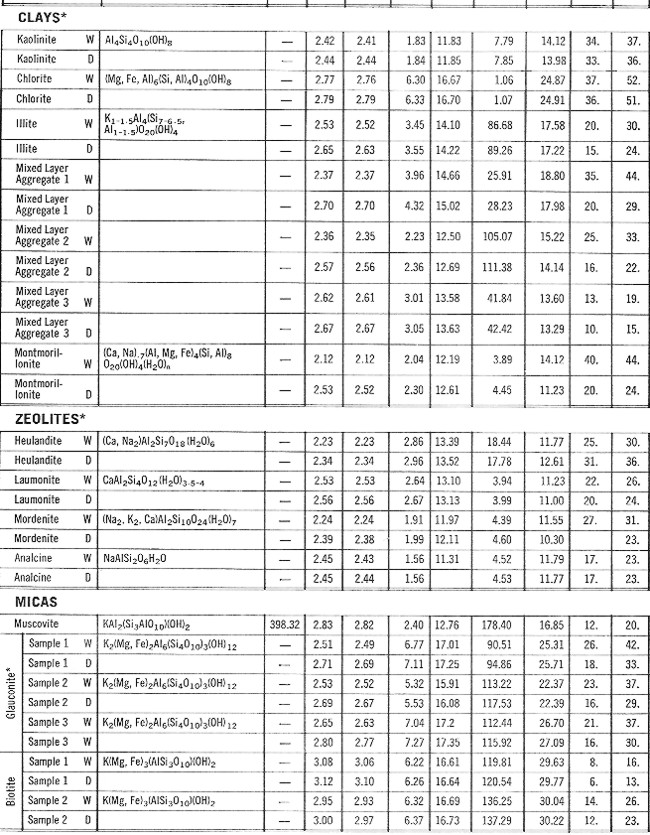
 ELASTIC PROPERTIES OF MINERALS
ELASTIC PROPERTIES OF MINERALS
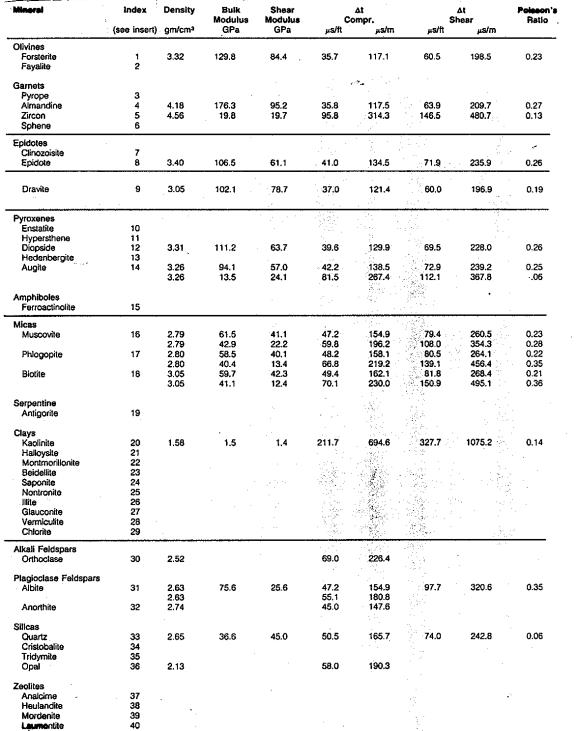
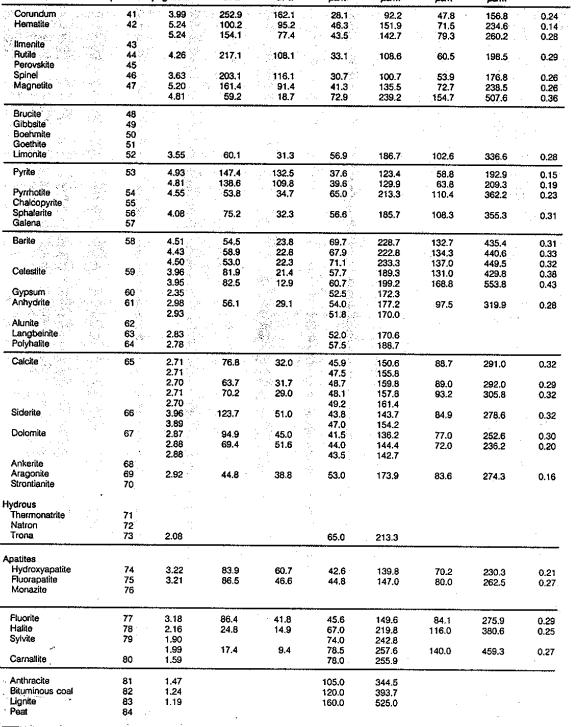
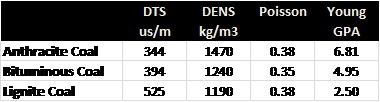 This table,
reproduced from the Schlumberger Chartbook, contains most of the
mineral data required for estimating elastic properties of non-porous
minerals. Since most rocks of economic interest in the oil and
gas industry are porous (not non-porous), this table may be of
limited value. Methods for calculating the correct values for the
porous case are shown HERE. Coal data
is pretty sparse in the literature - a brief summary is listed at
right. This table,
reproduced from the Schlumberger Chartbook, contains most of the
mineral data required for estimating elastic properties of non-porous
minerals. Since most rocks of economic interest in the oil and
gas industry are porous (not non-porous), this table may be of
limited value. Methods for calculating the correct values for the
porous case are shown HERE. Coal data
is pretty sparse in the literature - a brief summary is listed at
right.
 Igneous and
Metamorphic Rock Properties
Igneous and
Metamorphic Rock Properties
Metamorphic rocks are conventional sedimentary rocks that have
been exposed to high heat and pressure. My personal experience is that density, neutron, sonic and photoelectric
values for metamorphic rocks are the same as the sedimentary equivalent although
this may not be universally true..
-
Contact metamorphism - changes in the rock due to heat
from nearby magma.
-
Regional metamorphism
- causes change through intense heat and pressure.
-
Hydrothermal metamorphism
- chemical changes in the rock due to the circulation
of hot liquids through the rock fractures.
-
Fault zone metamorphism
- metamorphic changes caused friction at fault movements.
The quality of the rock is based on the amount of heat and
pressure applied to it during the metamorphic processes.
Changes That
Occur during metamorphism are:
-
Re
crystallization - occurs when small crystals join together to create
larger crystals of the same mineral.
-
Neomorphism - new minerals are created from the original mineral
composition.
-
Metamorphism - new minerals are created by gaining or losing
chemicals.
Specific sedimentary rocks
become specific metamorphic rocks, as shown below:
|
Parent
Rock |
New
Rock |
|
Sandstone
|
Quartzite |
|
Limestone, Dolomite |
Marble |
|
Basalt |
Schist or Amphibolite |
|
Shale |
Slate |
|
Granite |
Schist |
|
Rhyolite |
Schist |
Igneous
rocks are classified in several ways – by composition, texture,
and method of emplacement. The composition (mineral mixture) determines
the log response. The texture determines the name used for the
mineral mixture, and the method of emplacement determines the
texture and internal porosity structure (if any).
Intrusive igneous
rocks are formed inside the earth. This type of igneous rock cools very
slowly and is produced by magma from the interior of the earth. They
have large grains, may contain gas pockets, and usually have a high
fraction of silicate minerals. Intrusions are called sills when
lying roughly horizontal and dikes when near vertical.
Extrusive igneous
rocks form on the surface of the earth from lava flows. These cool
quickly. They have small grains and contain little to no gas.
Both intrusive and extrusive rocks may contain natural
fractures from contraction while cooling, and may have carried non-igneous
rocks with them, called xenoliths.
Intrusive rocks may alter the rocks above and below them by
metamorphosing (baking) the rock near the intrusion. Extrusives only
heat the rock below them, and may not cause much alteration due to
rapid cooling. Extrusives can be buried by later sedimentation, and
are difficult to distinguish from intrusives, except by their
chemical composition and grain size.
The mineral composition of an igneous rock depends on where
and how the rock was formed. Magmas around the world have different
mineral make up.
Felsic igneous rocks are light in color and are mostly made
up of feldspars and silicates. Common minerals found in felsic rock
include quartz, plagioclase feldspar, potassium feldspar
(orthoclase), and muscovite. They may contain up to 15% mafic
mineral crystals and have a low density.
Mafic igneous rocks are dark colored and consist mainly of
magnesium and iron. Common minerals found in mafic rocks include
olivine, pyroxene, amphibole, and biotite. They contain about 46-85%
mafic mineral crystals and have a high density.
Ultramafic igneous rocks are very dark colored and contain higher amounts of the
same common minerals as mafic rocks. They contain about 86-100%
mafic mineral crystals.
Intermediate igneous rocks are between light and dark colored. They share minerals
with both felsic and mafic rocks. They contain 15 to 45% mafic
minerals.
Plutonic
and volcanic rocks generally have very low porosity and permeability.
Natural fractures may enhance porosity by allowing solution of
feldspar grains. Some examples with average porosity as high as
17% are known.
Tuffs and tuffaceous
rocks have high total
porosity because of vugs or vesicles in a glassy matrix. This is
most common in pyroclastic deposits. Interparticle porosity may also exist. Some effort has to be made
to separate ineffective microporosity from the total porosity.
Pumice (a form of tuff) has enough ineffective porosity to allow
the rock to float! When
other minerals fill the vesicles by precipitation, the tuff is
called a zeolite.
|
IGNEOUS
ROCK CLASSIFICATION |
|
Plutonic |
Volcanic |
Pyroclastic |
|
Gamma
Ray |
|
Coarse
Crystalline |
Fine
Crystalline |
Glassy |
Silica
Content |
Density |
|
Quartzite |
|
|
Highest |
Lowest |
|
Granite |
Rhyolite |
Rhyolite
Tuff |
|
|
|
Granodioite |
Dacite |
Dacite
Tuff |
|
|
|
Quartzdiorite |
Andesite |
Andesite
Tuff |
|
|
|
Diorite |
Basalt |
Zeolite
Tuff |
|
|
|
Gabbro |
Dolerite |
|
|
|
|
Disabase |
|
|
|
|
|
Dunite |
|
|
Lowest |
Highest |
|
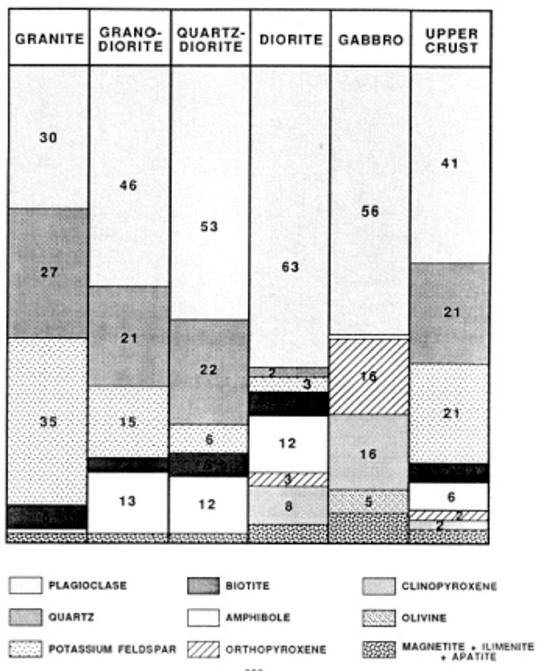
Typical igneous rock mineral composition
The
numerical data below has worked well in igneous reservoirs using
standard lithology models given earlier in this chapter (Mlith-Nlith,
DENSma-Uma, etc).
|
MATRIX
PROPERTIES FOR IGNEOUS ROCKS |
| |
DENSMA |
PE |
UMA |
PHINMA |
DTC_MA |
DTS_MA |
| Quartzite |
2.65 |
1.82 |
4.82 |
0.0 |
55.0 |
101.2 |
| Granite |
2.65 |
2.70 |
7.00 |
1.0 |
50.8 |
82.7 |
| Granodiorite |
2.72 |
3.25 |
8.75 |
2.0 |
55.0 |
97.1 |
| Quartzdiorite |
2.81 |
3.56 |
9.91 |
3.5 |
57.0 |
89.9 |
| Diorite |
2.85 |
3.95 |
11.0 |
4.0 |
57.1 |
96.8 |
| Gabbro |
2.94 |
4.80 |
13.3 |
5.0 |
42.4 |
90.1 |
| Diabase |
2.98 |
|
|
|
44.6 |
85.8 |
| Dunite |
3.29 |
3.40 |
11.2 |
4.0 |
38.2 |
76.9 |
The
table is in English units. If you work in Metric units, divide
neutron values by 100, multiply density by 1000, and multiply
sonic by 3.281.
All
these values have a moderate range (+/- 10%) and some tuning may
be necessary. Don’t forget to metricate the numbers if needed.
Use these matrix values in the matrix density or PE crossplots
shown earlier.
Since a typical log suite can solve for 3 or 4 minerals at best,
you need to chose the dominant minerals and zone your work carefully.
If you have additional useful log curves, you might try for more
minerals or set up several 4 mineral models in a probabilistic
solution. A good core or sample description will help you choose
a reasonable mineral suite.
Sometimes
lithology is determined by triggers. For example, where basalt
beds are interspersed between conventional granites or quartzites,
it is easy to use the PE or density logs to trigger basalt,
leaving the remaining minerals to be defined by a two or three
mineral model. This approach is widely used in sedimentary
sequences to trigger anhydrite, coal, or salt.
Two
crossplots are useful for rock identification in metamorphic
rocks, as shown
below. The math for running two and
three mineral models was shown earlier in this Chapter.
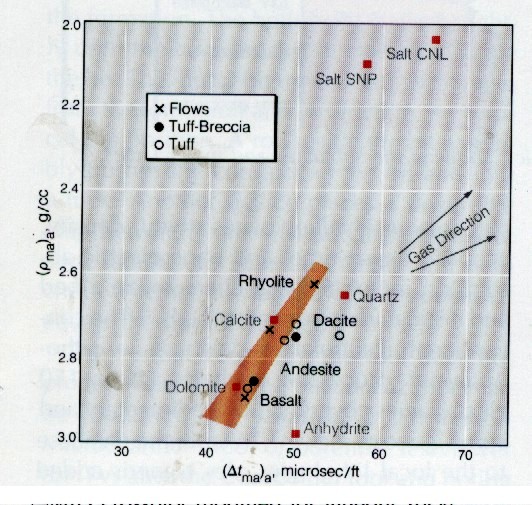
DENSMA vs DELTMA Plot
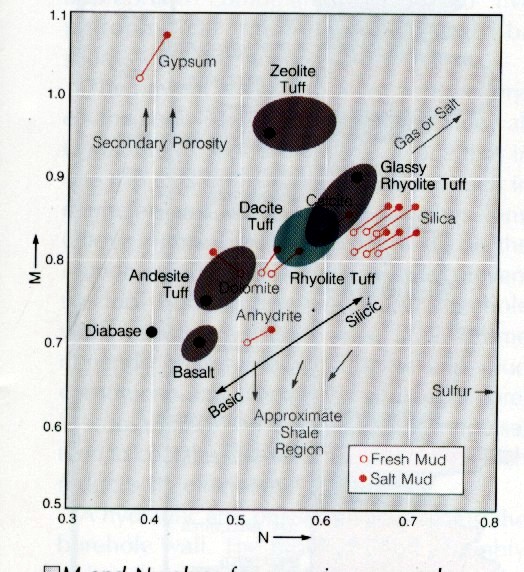
Mlith vs Nlith Plot
for Igneous Rocks
|






 This table,
reproduced from the Schlumberger Chartbook, contains most of the
mineral data required for estimating elastic properties of non-porous
minerals. Since most rocks of economic interest in the oil and
gas industry are porous (not non-porous), this table may be of
limited value. Methods for calculating the correct values for the
porous case are shown
This table,
reproduced from the Schlumberger Chartbook, contains most of the
mineral data required for estimating elastic properties of non-porous
minerals. Since most rocks of economic interest in the oil and
gas industry are porous (not non-porous), this table may be of
limited value. Methods for calculating the correct values for the
porous case are shown 



