 BASIC CHEMISTRY
BASIC CHEMISTRY
A molecule is a sufficiently stable, electrically neutral, assemblage
of two or more atoms held together by strong chemical bonds.
A
chemical compound is a combination of two or more elements or
molecules, such
as quartz, a combination of silicon and oxygen, or dolomite, a
compound of calcium, magnesium, carbon, and oxygen. Water
is a compound of hydrogen and oxygen.
There
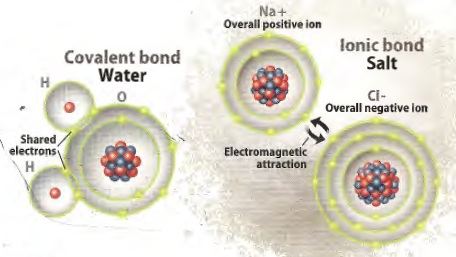
are two basic kinds of compounds: ionic and covalent. Ionic
compounds are held together by electromagnetic attraction between
positive and negative ions, for example NaCl (sodium chloride,
halite, rock salt) or CaCO3 (calcium carbonate, calcite,
limestone).
Covalent compounds are held together by sharing electrons, such as
H2 (hydrogen), O3 (ozone), CH4 (methane), H2O (water).
The
sharing of free electrons in metals, called metallic bonding, is
similar in concept to ionic bonding. Many compounds have bonding
that is a combination of covalent and ionic.
A
mixture is a physical combination of a minimum of two elements
or compounds. No chemical reactions take place between the mixed
components. For example, sandstone is a mixture of quartz,
water and/or oil and/or gas, and/or other constituents such as
clay, silt, or any other rock mixtures. Salt dissolved in water
is also a mixture.
When
a compound is formed from two or more elements, the volume of
the resulting molecule may be more or less than the original
components. However, the total weight or mass, will not change,
providing all gases formed, if any, are retained.
When
a physical mixture is created, such as sand grains and water,
the volume of the resulting mixture is the sum of the volumes
of the original components, provided any gases involved,
such as air between sand grains, are retained, and held at a constant
temperature and pressure. The mass again will remain the sum of
the masses of the individual components.
 VALENCE, ELECTRON CONFIGURATIONS, and BONDING
VALENCE, ELECTRON CONFIGURATIONS, and BONDING
Valence
electrons are the outermost electrons of an atom, which are
important in determining how the atom reacts chemically with other
atoms. Atoms with a complete shell of valence electrons are chemically inert. Atoms with one or
two valence electrons more than a closed shell are highly reactive
because the extra electrons are easily removed to form positive
ions. Atoms with one or two valence electrons less than a closed
shell are also highly reactive because of a tendency either to gain
the missing electrons and form negative ions, or to share electrons
and form covalent bonds.
Valence electrons have the
ability to absorb or release energy
in the form of photons. This gain or loss of energy can trigger an
electron to move (jump) to another shell or even break free from the
atom and its valence shell. When an electron absorbs energy in the
form of one or more photons, then it moves to a more outer shell,
depending on the amount of energy gained. When an electron loses
energy (photons), then it moves to a more inner shell.
The number of electrons in an
atom's outermost valence shell governs its bonding behavior.
Therefore, elements with the same number of valence electrons are
grouped together in the periodic table of the elements. As a general
rule, atoms of main group elements (except hydrogen and helium) tend
to react to form a "closed" or complete shell, corresponding to an
s2p6 electron configuration. This tendency is called the octet rule
since the bonded atom has or shares eight valence electrons.
The most reactive metallic
elements are the alkali metals of Group 1, for example sodium (Na)
and potassium (K) whose atoms each have a single valence electron.
This is easily lost to form a positive ion (cation) with a closed
shell (Na+ or K+), during the formation of an ionic bond which
provides the necessary ionization energy. The alkaline earth metals
of Group 2, for example magnesium, are somewhat less reactive since
each atom must lose two valence electrons to form a positive ion
with a closed shell such as Mg2+.
Nonmetal atoms tend to attract
additional valence electrons to attain a full valence shell. This
can be achieved one of two ways: an atom can either share electrons
with neighboring atoms, a covalent bond, or it can remove electrons
from other atoms, an ionic bond. The most reactive non-metals are
the halogens such as fluorine (F) and chlorine (Cl), which have
electron configurations s2p5 and require only one additional valence
electron for a closed shell. To form an ionic bond, a halogen atom
can remove an electron from an other atom to form an anion
(F-, Cl-, etc.). To form a covalent bond, one electron from the
halogen and one electron from another atom form a shared pair. For
example in the molecule H-F, the line represents a shared pair of
valence electrons, one from H and one from F.
In these simple cases where the
octet rule is obeyed, the valence of an atom equals the number of
electrons gained, lost or shared to form the stable octet. However
there are also many molecules which are exceptions, and for which
the valence is less clearly defined.
The valence electrons are also
responsible for the electrical conductivity of elements, which may
be divided into metals, nonmetals, and semiconductors or metalloids.
Metals or metallic elements are
elements with high electrical conductivity in the solid state. In
each row of the periodic table the metals occur to the left of the
nonmetals and thus have fewer valence electrons. The valence
electrons which are present have small ionization energies, and in
the solid state they are relatively free to leave one atom and move
to its neighbour. These “free electrons” can move under the
influence of an electric field and their motion constitutes an
electric current. They are therefore responsible for the electrical
conductivity of the metal. Copper, aluminium, silver and gold are
examples of good conductors used widely in industry.
Nonmetallic elements have low
electrical conductivity and act as insulators. They are found to the
right of the periodic table with valence shells which are at least
half full (except for boron). Their ionization energies are large so
that electrons cannot leave an atom easily when an electric field is
applied, and they conduct only very small electric currents.
Examples of solid elemental insulators are diamond (an elemental
form of carbon) and sulphur.
Solid compounds containing metals
can also be insulators if the valence electrons of the metal atoms
are used to form ionic bonds. For example, although elemental sodium
is a metal, solid sodium chloride is an insulator because the
valence electron of sodium is transferred to chlorine to form an
ionic bond and cannot move easily in an electric field.
Semiconductors have an electrical
conductivity intermediate between metals and nonmetals, and also
differ from metals in that their conductivity increases with
temperature. The typical elemental semiconductors are silicon and
germanium with four valence electrons each. Their properties are
best explained using band theory, as a consequence of a small energy
gap between a valence band which contains the valence electrons at
absolute zero, and a conduction band to which valence electrons are
excited by thermal energy.
|

