|
 Formation Water Resistivity BASICS
Formation Water Resistivity BASICS
Most methods for computing water saturation require knowledge
of formation water resistivity at the formation temperature, so
it is a necessary evil along our step-by-step path to find out how
much oil and gas is in the ground.
Sodium chloride makes up the majority of the dissolved solids in
hydrocarbon reservoirs, but numerous other compounds may be present.
When salts dissolve, they break down into their ions, such as Na, Cl,
Mg, SO4, K, Ca, and many others. Pure water has near infinite
resistivity; it is these ions that make water conductive.
Water resistivity decreases with increased salinity, and for
a given salinity, water resistivity also decreases with
increased temperature. At any given temperature, there is a
maximum salinity that can be achieved, above which salt crystals
will begin to precipitate. In non-geothermal reservoirs, this limit
is between 225,000 and 325,000 parts per million (ppm) total
dissolved solids (TDS).
Produced water samples can be analyzed in the laboratory for
their chemical composition and water resistivity. Less accurate
water resistivity values can be measured at the well site or
can sometimes be derived from well log data. In all cases,
manipulation of the RW values to account for temperature
will probably be needed. Conversion between salinity and
resistivity and vice versa is commonly needed. And of
course some method for estimating formation and surface temperatures will
be required.
Other pages in this Chapter develop
the math to handle all these critical factors.
 Laboratory Water Analysis REPORTS
Laboratory Water Analysis REPORTS
Laboratories
usually measure from 9 to 15 of the individual ions in a water
sample, recorded in milligrams/litre (mg/l) or grams/cubic meter
(g/m3). These two sets of units are equivalent: 1 mg/l =
1 g/m3.
At low to moderate concentrations, one mg/l is very
close to 1 part per million (ppm) so mg/l and ppm tend to be used
interchangeably.
In older reports, results were quoted in
grains per gallon (gpg) One grain per US gallon equals 17.1 mg/l or
approximately 17.1 ppm.
Drill stem test
or produced water recoveries in your well, or from nearby wells,
are analyzed for chemical content and water resistivity in the laboratory.
A sample analysis is shown below.
The
chemical analysis is recorded in milligrams per kilogram (mg/kg), or milligrams per
liter (mg/l).
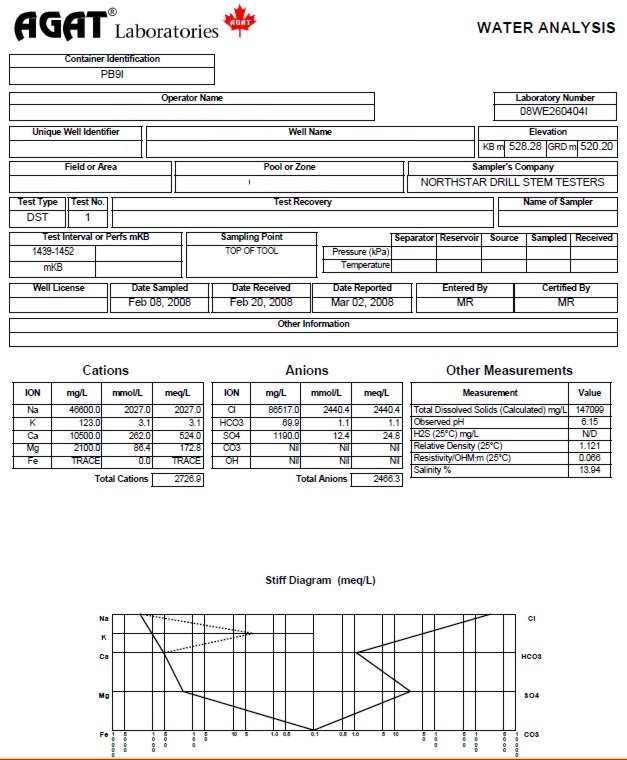
Water analysis report from a drill
stem test recovery, showing chemical analysis, calculated and
measured water resistivity, and Stiff diagram of chemical analysis.
|

