|
 WATER SALINITY and Total
dissolved
solids
WATER SALINITY and Total
dissolved
solids
The salinity
of a water sample can be described numerically in several
different ways, each with a confusing set of measurement
units. This page covers the summation of water chemistry
analysis data and conversions between total dissolved solids
(TDS), equivalent NaCl content, and chloride content.
Conversion of salinity to water resistivity is covered on
the next Section in this Chapter.
 Equivalent NaCl
Water Salinity
from Water Analysis
Equivalent NaCl
Water Salinity
from Water Analysis
The
resistivity of a water sample can be calculated from its
chemical analysis. To do this, an equivalent NaCl concentration
must be determined based on the ionic activity of each ion.
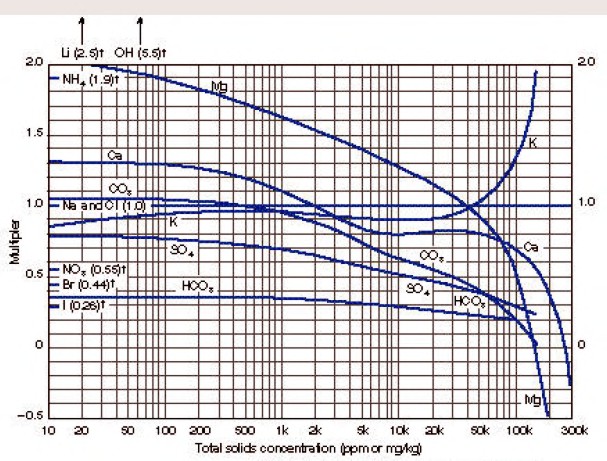
Enter chart with total solids concentration of the sample in ppm
(mg/kg) to find weighting factors for each ion present. The
concentration of each ion is multiplied by its weighting factor, and
the products for all ions are summed to obtain equivalent NaCl
concentration.
The math is
pretty simple:
1: TDS = SUM (IONi)
2:
WSe = SUM (IONi * FACTRi)
Where:
TDS = total dissolved solids (ppm)
IONi = ion concentration of ith component (ppm)
FACTRi = multiplier factor for ith component (ppm)
WSe = equivalent NaCl concentration (ppm)
 NUMERICAL EXAMPLE:
NUMERICAL EXAMPLE:
Assume formation-water sample analysis
460 ppm Ca,
1400 ppm SO4
19,000 ppm Na plus Cl.
Total dissolved solids concentration
TDS = 460 + 1400 + 19,000 = 20,860 ppm
Entering the chart with this total solids concentration
Ca multiplier = 0.81
SO4 multiplier = 0.45
Na+CL multiplier = 1.00
Equivalent NaCl concentration = 460 ´ 0.81 + 1400 ´ 0.45 + 19,000 ´
1.0 = 20,000 ppm.
 Water Salinity from
Chloride Content Water Salinity from
Chloride Content
Sometimes salinity is reported at the well site in ppm Chlorides
instead of ppm NaCl equivalent.
6: WSa = Ccl * 1.645
Where:
Ccl = water salinity (ppm Cl)
WSa = water salinity (ppm NaCl)
 COMMENTS:
COMMENTS:
Use this relationship when chloride content of the water sample
is known. Usually Cl content is derived at the
well site from a drill stem test recovery. It is useful as a
first approximation until the water sample is analyzed more
accurately at a laboratory. The relationship is for pure NaCl
solutions and the factor may be higher or lower if other ions
are present.
 NUMERICAL
EXAMPLE:
NUMERICAL
EXAMPLE:
1. Chloride concentration to salinity.
WS = 11,600 ppm Cl * 1.645 = 19,000 ppm NaCl
|

