|
 Lithium BASICS
Lithium BASICS
Petro-lithium is present in oilfield produced water
and in moderate to highly saline water zones in sedimentary
basins. Well logs cannot identify lithium in oilfield brines
(at least not yet) but they do tell us a lot about the water
salinity, pore volume filled with that water, and other
pertinent information about the reservoir or aquifer. This
article describes near surface and deep sources of lithium,
how it is extracted from brines, as well as the mining
technique which until recently was the major source of the
world’s lithium.
Lithium (Li) is a soft,
silvery-white alkali metal. Under standard conditions, it is
the lightest metal and the least dense solid element. Like
all alkali metals, lithium is highly reactive and flammable,
and must be stored in vacuum, inert atmosphere, or inert
liquid such as purified kerosene or mineral oil.
It never occurs freely in nature, but mostly in ionic
compounds, such as pegmatitic minerals (spodumene, and to
lesser extent, amblygonite, lepidolite,
and petalite), which were
once the main source of lithium. Extraction was a classical
hard rock mining operation in which the ore was heated,
crushed, and leached to obtain stable lithium-rich
compounds.
Since about 1990, surface and near-surface brines in lakes,
playa deposits, and salt flats have become major sources of
lithium compounds.
Soon oilfield produced water, oilfield water zones, and
medium temperature geothermal projects will be capturing
lithium from these higher salinity water flows.
Lithium and its compounds have many industrial uses
including heat-resistant glass and ceramics, lithium grease
lubricants, flux additives for iron, steel, and aluminum
production, and lithium or lithium-ion batteries. These uses
consume more than three-quarters of lithium production
(2020), and will continue to increase rapidly as the World’s
vehicle fleet is electrified..
Lithium reserves and resources are measured in
metric tonnes Li metal equivalent. Hard rock ore grade is
reported in percent Li2O, similar to potash ore grade in
percent K2O. An average ore grade for a hard rock mine might
be 2.4%. In brines, quality is graded in parts per million (ppm
or mg/liter) Li+ ions – 500 ppm represents a fairly high
concentration in an oilfield brine or a moderate value for a
near-surface brine deposit.
USGS and other studies show the lithium resource is
available for projected needs but extraction may lag demand.
Petrophysics, with other geosciences, will play a major role
in quantifying reservoir volumes, water quality, and flow
capacity that will help to assess the economics of these
projects.
 LITHIUM EXTRACTION FROM
HARD ROCK ORES
LITHIUM EXTRACTION FROM
HARD ROCK ORES
Ore from hard rock mining
of pegmatic minerals is heated to 1200K and crushed, The
minerals are combined with sulphuric acid and sodium
carbonate which causes the aluminum and iron to precipitate
from the ore. Sodium carbonate is added to the lithium
products which causes the lithium to precipitate out in the
form of lithium carbonate (Li2CO3).
Hydrochloric acid is added to the Lithium carbonate to form
lithium chloride.
 LITHIUM EXTRACTION FROM
SURFACE BRINES
LITHIUM EXTRACTION FROM
SURFACE BRINES
A large fraction of the
world's current lithium is produced by evaporation of brine
in ponds. This process is time-consuming, but is also
inexpensive compared to other methods. The salt-rich waters
are pumped from the ground and start to evaporate through
solar energy. This process can take several months, up to
two years. First, potassium is harvested. Then when the
lithium compounds reaches a suitable concentration, they are
harvested and brought to a plant. Unwanted waste is filtered
out, then the concentrate is treated with sodium carbonate,
to create lithium carbonate. Finally, the unwanted waste is
pumped back into the ground.
Seawater has only 0.2 ppm Li so it is not considered a
credible source of economic lithium.
 LITHIUM EXTRACTION FROM
DEEP BRINES
LITHIUM EXTRACTION FROM
DEEP BRINES
Lithium from oilfield
produced brines (50 – 500+ ppm Li+), co-produced with oil or
natural gas, and from deep high salinity oilfield water
zones is in its infancy but is much more environmentally
friendly than destroying salt flats or pegmatic mountains.
Note that oilfield produced water is sometimes called
oilfield waste water, not to be confused with municipal
waste water, which is a very different thing. Oilfield waste
water is injected back into the reservoir where it came
from; municipal waste water may or may not be treated and is
fed into rivers or oceans.
Co-production of lithium with medium temperature geothermal
energy projects in sedimentary basins looks very attractive.
As in the oilfield case, the water is from deep saline zones
and is already being pumped to a disposal well.
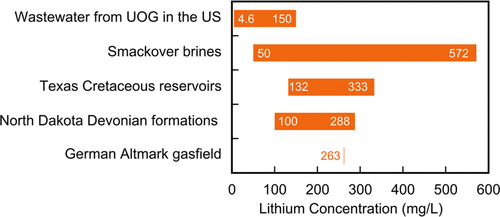
Lithium concentration of various produced oilfield brines (Kumar et al
2019)
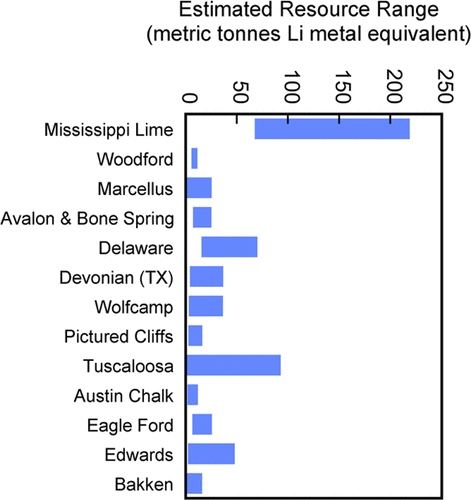
Lithium resources of various produced oilfield brines (Kumar et al
2019)
Fox Creek and Valleyview in Canada have 362 000 and 385 000 metric
tonnes of Li metal equivalent, respectively, while the Smackover
Formation in the U.S. has 750 000 metric tonnes.
Lithium may be extracted from the oilfield produced water and
geothermal water flowing to the disposal well using an adsorption,
membrane-based processes, and electrolysis-based
systems. These and other approaches are in the pilot project stage
(2022).
Extraction of lithium from oilfield and geothermal brines enables
domestic production without relying on South American sources and
Chinese refining.
Apparently 100 ppm Li+ can make extra cash, since the water is
already being separated and pumped to the disposal site. Repurposing
deep wells that have reached end of life as oil or gas wells will
need higher concentrations of Li+ to pay out the pumping costs.
Time to drag out all those old water chemistry reports, or to take
new water samples from produced water to see what we own.
There will probably be some mineral-rights and regulatory issues to
resolve with various agencies and land owners. Further, some
innovative approaches are needed to test saline water zones for
lithium concentration before a well is prematurely abandoned.
Transforming unwanted oil wells into “green energy” sources is very
appealing.
REFERENCES
1. Lithium Recovery from Oil and Gas Produced Water
Amit Kumar,
Hiroki Fukuda, Alan Hatton, and John
H. Lienhard
https://doi.org/10.1021/acsenergylett.9b00779
2. Lithium Technical Data
Various Wikipedia Pages
|




