|
 COAL BASICS
COAL BASICS
Coal is a term
used to describe a wide range of organic compounds composed of
macerals (as opposed to minerals). A maceral is a component of
coal or oil shale. The term 'maceral' in reference to coal is
analogous to the use of the term 'mineral' in reference to
igneous or metamorphic rocks. Examples of macerals are
inertinite, vitrinite and liptinite. Macerals are forms of
kerogen with varying carbon, oxygen, and hydrogen content. The
kerogen in coals are mostly type 3 and 4 kerpgen.
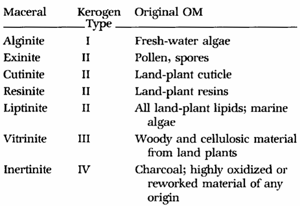
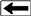 Coal
macerals are Type 3 and Type 4 Kerogens. Coals are often described
by their common names instead of maceral type. Coal
macerals are Type 3 and Type 4 Kerogens. Coals are often described
by their common names instead of maceral type.
 Bituminous coal
is an organic sedimentary rock formed by diagenetic and
submetamorphic compression of peat bog material. Il has been
compressed and heated so that its primary constituents are macerals.
The carbon content of bituminous coal is around 60 to 80%; the rest
is composed of water, air, hydrogen, and sulfur, which have not been
driven off from the macerals. Bituminous coal or black coal is
relatively soft, containing a tarlike substance called bitumen. It
is of higher quality than lignite coal but of poorer quality than
anthracite coal. Bituminous coal
is an organic sedimentary rock formed by diagenetic and
submetamorphic compression of peat bog material. Il has been
compressed and heated so that its primary constituents are macerals.
The carbon content of bituminous coal is around 60 to 80%; the rest
is composed of water, air, hydrogen, and sulfur, which have not been
driven off from the macerals. Bituminous coal or black coal is
relatively soft, containing a tarlike substance called bitumen. It
is of higher quality than lignite coal but of poorer quality than
anthracite coal.
  Lignite,
often referred to as brown coal, is a soft brown fuel with
characteristics that put it somewhere between coal and peat. It is
considered the lowest rank of coal, used almost exclusively as a
fuel for steam-electric power generation. Lignite has a carbon
content of around 25 to 35%, a high inherent moisture content
sometimes as high as 66%, and an ash content ranging from 6% to 19%
compared with 6% to 12% for bituminous coal. Lignite,
often referred to as brown coal, is a soft brown fuel with
characteristics that put it somewhere between coal and peat. It is
considered the lowest rank of coal, used almost exclusively as a
fuel for steam-electric power generation. Lignite has a carbon
content of around 25 to 35%, a high inherent moisture content
sometimes as high as 66%, and an ash content ranging from 6% to 19%
compared with 6% to 12% for bituminous coal.
 Anthracite is a hard, compact variety of mineral
coal that has a high luster. It has the highest carbon content,
between 92% and 98%, and contains the fewest impurities of all
coals, despite its lower calorific content. Anthracite is the most
metamorphosed type of coal. The term is applied to coals which do
not give off tarry or other hydrocarbon vapours when heated below
their point of ignition. Anthracite is a hard, compact variety of mineral
coal that has a high luster. It has the highest carbon content,
between 92% and 98%, and contains the fewest impurities of all
coals, despite its lower calorific content. Anthracite is the most
metamorphosed type of coal. The term is applied to coals which do
not give off tarry or other hydrocarbon vapours when heated below
their point of ignition.
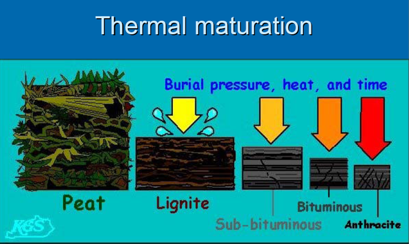
Coal rank depends on thermal maturity
Coal rank is determined by
the BTU heating content or by the fixed carbon content, on a dry,
ash-free basis.
 Proximate analysis
Proximate analysis
Proximate analysis of coal is a simple
laboratory method for determining the components of coal, obtained when the coal sample is heated (pyrolysis) under
specified conditions. The coal sample is extracted from a core
and placed quickly in a canister to preserve as much gas as
possible.
As defined by ASTM D 121, proximate
analysis separates the coal into four groups:
1. moisture,
2. volatile matter, consisting of gases and vapors driven off
during pyrolysis,
3. fixed carbon, the nonvolatile fraction of coal
4. ash, the inorganic residue remaining after combustion.
Fixed carbon is also called carbon, dry coal, pure coal, or
dry ash-free coal. The latter term is the most descriptive - dry
ash-free is often abbreviated as "daf" or "DAF".
 Moisture
is an important property of coal, as all coals are mined wet.
Groundwater and other extraneous moisture is known as adventitious
moisture and is readily evaporated. Moisture held within the coal
itself is known as inherent moisture and is analyzed quantitatively.
Adventitious moisture is removed in the lab by evaporation in air. Moisture
is an important property of coal, as all coals are mined wet.
Groundwater and other extraneous moisture is known as adventitious
moisture and is readily evaporated. Moisture held within the coal
itself is known as inherent moisture and is analyzed quantitatively.
Adventitious moisture is removed in the lab by evaporation in air.
Moisture may occur in four possible forms within coal:
1. surface moisture: water held on
the surface of coal particles or macerals
2. hydroscopic moisture:
water held by capillary action within the micro-fractures of the coal
3. decomposition moisture: water held within the coal's decomposed
organic compounds
4. mineral moisture: water which comprises part of
the crystal structure of hydrous silicates such as clays
Total
moisture is analyzed by loss of mass between an air-dried sample and
the sample after driving off the inherent moisture with heat. This is achieved by any of the following
methods;
1. heating the coal with toluene
2. drying in a minimum free-space oven at 150 °C (302 °F) within a
nitrogen atmosphere
3. drying in air at 100 to 105 °C (212 to 221 °F)
Methods 1 and 2 are suitable with low-rank coals but method 3 is
only suitable for high-rank coals as free air drying low-rank coals
may promote oxidation.
 Volatile matter
in coal refers to the components of coal, except for moisture, which
are liberated at high temperature in the absence of air. This is
usually a mixture of short and long chain hydrocarbons, aromatic
hydrocarbons, and some sulfur. In Australian and British
laboratories, this involves heating the coal sample to 900 ±
5 °C (1650 ±10 °F) for 7 minutes in a cylindrical silica crucible in
a muffle furnace. American procedures involve heating to
950 ± 25 °C (1740 ± 45 °F) in a vertical platinum crucible. These
two methods give different results and thus the method used must be
stated. Volatile matter
in coal refers to the components of coal, except for moisture, which
are liberated at high temperature in the absence of air. This is
usually a mixture of short and long chain hydrocarbons, aromatic
hydrocarbons, and some sulfur. In Australian and British
laboratories, this involves heating the coal sample to 900 ±
5 °C (1650 ±10 °F) for 7 minutes in a cylindrical silica crucible in
a muffle furnace. American procedures involve heating to
950 ± 25 °C (1740 ± 45 °F) in a vertical platinum crucible. These
two methods give different results and thus the method used must be
stated.
 Fixed carbon
content of the coal is the carbon found in the material which is
left after volatile materials are driven off. This differs from the
ultimate carbon content of the coal because some carbon is lost in
hydrocarbons with the volatiles. Fixed carbon is used as an estimate
of the coke yield from a sample of coal.
Fixed carbon is determined by subtracting the mass of volatiles,
determined above, from the original mass of
the coal sample. Fixed carbon
content of the coal is the carbon found in the material which is
left after volatile materials are driven off. This differs from the
ultimate carbon content of the coal because some carbon is lost in
hydrocarbons with the volatiles. Fixed carbon is used as an estimate
of the coke yield from a sample of coal.
Fixed carbon is determined by subtracting the mass of volatiles,
determined above, from the original mass of
the coal sample.
 Ash
content of coal is the non-combustible residue left after coal is
burnt. It represents the bulk mineral matter after carbon, oxygen,
sulfur and water (including from clays) has been driven off during
combustion. Analysis is fairly straightforward, with the coal
thoroughly burnt and the ash material expressed as a percentage of
the original weight. Ash
content of coal is the non-combustible residue left after coal is
burnt. It represents the bulk mineral matter after carbon, oxygen,
sulfur and water (including from clays) has been driven off during
combustion. Analysis is fairly straightforward, with the coal
thoroughly burnt and the ash material expressed as a percentage of
the original weight.
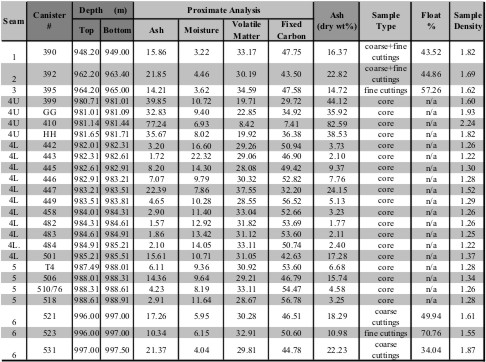
Example of Proximate Analysis of
several coal seams - data is in Weight %
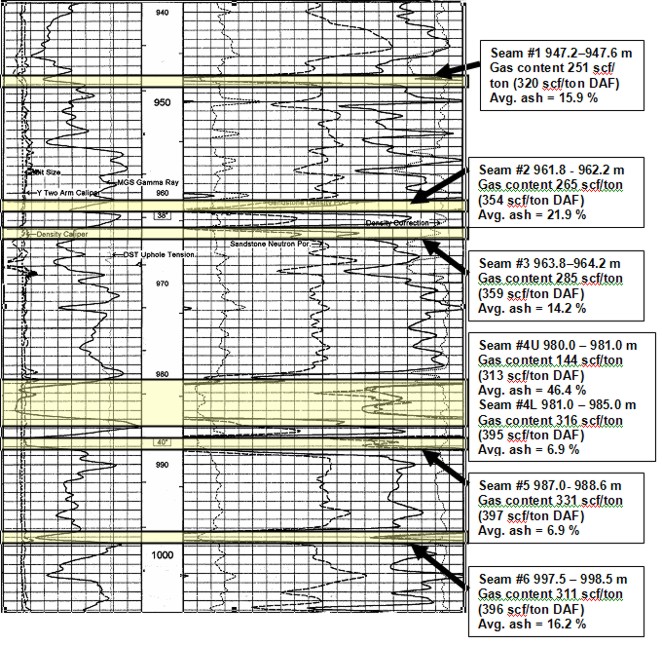
Well log showing location of coal
layers analyzed by proximate analysis. Log curves are GR, CAL, PE,
neutron, density, density correction.
 Float
Sink Analysis is used to separate non-coal cavings
from cuttings samples. The crushed material is placed in a liquid
with a density of 1.75 g/cc. The coal fraction is floated off and
the non-coal sinks and is removed. Some mineral (ash) in the coal
may sink, reducing the apparent ash content.
By
comparing the ash analysis to the float sink analysis with that from
core analysis, the gas contents can be normalised to reflect true
ash contents of the coal cuttings.
Float
Sink Analysis is used to separate non-coal cavings
from cuttings samples. The crushed material is placed in a liquid
with a density of 1.75 g/cc. The coal fraction is floated off and
the non-coal sinks and is removed. Some mineral (ash) in the coal
may sink, reducing the apparent ash content.
By
comparing the ash analysis to the float sink analysis with that from
core analysis, the gas contents can be normalised to reflect true
ash contents of the coal cuttings.
 Vitrinite is the most common component of coal.
It is also abundant in kerogen, derived from the same
biogenic precursors as coals, namely land plants and humic peats.
Vitrinite forms diagenetically by the thermal alteration of lignin
and cellulose in plant cell walls. It is therefore common in
sedimentary rocks that are rich in organic matter, such as shales
and marls with a terrigenous origin.
Conversely, carbonates, evaporites, and well-sorted sandstones have
very low vitrinite content. Vitrinite is absent in pre-Silurian
rocks because land plants had not yet evolved. Vitrinite is the most common component of coal.
It is also abundant in kerogen, derived from the same
biogenic precursors as coals, namely land plants and humic peats.
Vitrinite forms diagenetically by the thermal alteration of lignin
and cellulose in plant cell walls. It is therefore common in
sedimentary rocks that are rich in organic matter, such as shales
and marls with a terrigenous origin.
Conversely, carbonates, evaporites, and well-sorted sandstones have
very low vitrinite content. Vitrinite is absent in pre-Silurian
rocks because land plants had not yet evolved.
 Vitrinite reflectance
was first studied by coal geologists attempting to determine the
thermal maturity, or rank, of coal beds. More recently, it is used
to study sedimentary organic matter from kerogen. It is sensitive
to temperature ranges that correspond to hydrocarbon generation (60
to 120°C). This means that, with a suitable calibration, vitrinite
reflectance can be used as an indicator of maturity in hydrocarbon
source rocks. Generally, the onset of oil generation is correlated
with a reflectance of 0.5 to 0.6% and the termination of oil
generation with reflectance of 0.85 to 1.1%
Vitrinite reflectance
was first studied by coal geologists attempting to determine the
thermal maturity, or rank, of coal beds. More recently, it is used
to study sedimentary organic matter from kerogen. It is sensitive
to temperature ranges that correspond to hydrocarbon generation (60
to 120°C). This means that, with a suitable calibration, vitrinite
reflectance can be used as an indicator of maturity in hydrocarbon
source rocks. Generally, the onset of oil generation is correlated
with a reflectance of 0.5 to 0.6% and the termination of oil
generation with reflectance of 0.85 to 1.1%
 VISUAL LOG ANALYSIS OF COAL Beds
VISUAL LOG ANALYSIS OF COAL Beds
The use of well logs for analyzing coal deposits dates back many
years. Most methods are based on a multi-mineral model which
solves for moisture, volatile components, fixed carbon, and ash.
These are the same components determined from coal cores or
sample chips by
proximate analysis.
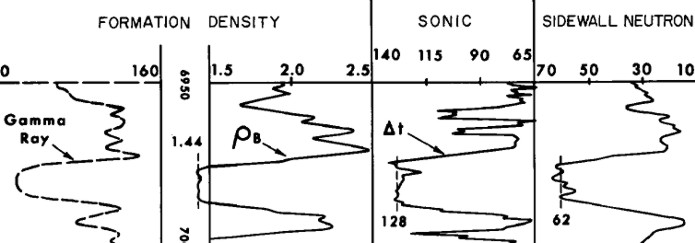
Visual analysis of logs for coal is relatively unambiguous.
High neutron porosity, high density porosity (low density), high
sonic, high resistivity, and clean gamma ray mean coal.
Thresholds on each curve are used to trigger a coal flag. Three
or more flags is a pretty good indication of the presence of
coal. Some coals are very dirty (shaly) so the gamma ray and
resistivity may not trigger.
 COAL ANALYSIS frOm LogS
COAL ANALYSIS frOm LogS
The use of well logs for analyzing coal deposits dates back many
years. Most methods are based on a multi-mineral model which
solves for moisture, volatile components, fixed carbon, and ash.
These are the same components determined from coal cores or
sample chips by proximate analysis.
One log
analysis model calculates a 3-mineral model from PE, density,
neutron, sonic crossplot methods and solves for the fraction of
lignite, bituminous coal, and anthracite. With this
breakdown, the coal matrix density can be determined, and the other
parameters follow from this value:
1: DENSMAcoal = Vlignite * 1.19 + Vbituminous * 1.34 +
Vanthracite * 1.47
An alternative method is a 3-mineral model using ash, fixed
carbon, and moisture. The GR is used to obtain Vclay, making a
4-mineral model relatively easy. Both models can be solved by
crossplots or the math shown elsewhere in this Handbook.
LOG
PARAMETERS FOR COAL TYPE ANALYSIS
DENSMA PHIN
DTC DTCMA PE Carbon
Oxygen Hydrogen
g/cc frac
us/ft us/m us/ft us/m
Wt% Wt% Wt%
Anthracite 1.47 0.41
105 345 48 157 0.16 95
1 2
Bituminous 1.24 0.60+ 120
394 44 144 0.17 82 10
5
Lignite 1.19
0.54 160 525 50 164 0.20
71 22 6
Peat 1.14
0.26 0.25
57 36 6
MATRIX PARAMETERS FOR 3-MINERAL MODEL - COAL COMPOSITION
DENSMA PHIN DTCMA PE
g/cc frac us/ft us/m
cu
Ash (Quartz) 2.65 0.00 55 182 1.8 Could vary if
other minerals (eg calcite) are also present
Ash (Clay) 2.18 - 2.65 0.25 80 250 3.5 Includes clay
bound water, varies with clay mineral
Carbon 1.19 - 1.47 0.60 120 394 0.2 Varies with
coal type (dry, ash-free value)
Water 1.00 1.00 200 656 0.1 Free water
or "moisture", excludes clay bound water
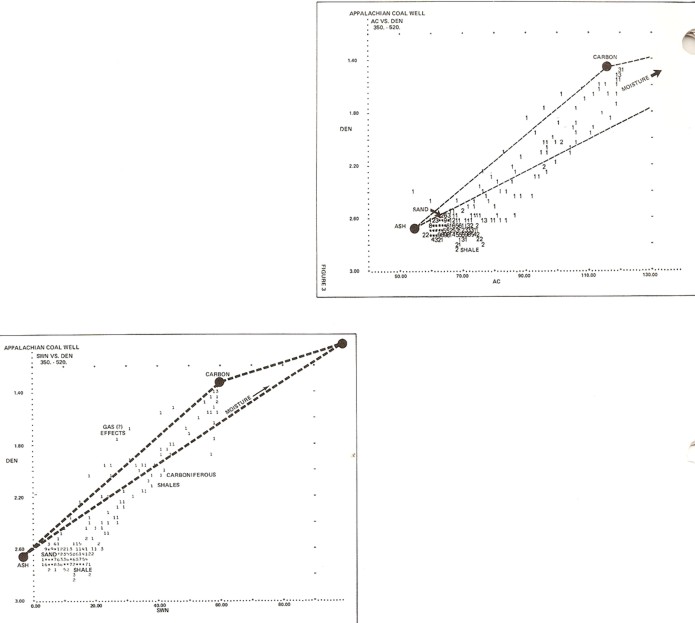
Density neutron crossplot for coal analysis (bottom left),
density sonic crossplot (top right). Data points show that the ash
in this coal is mostly clay (log data falls to the right of the
quartz point).
The mineral end points are not firm, so some experimentation
and sample descriptions are needed. If a 3-mineral model is not
possible, the analyst must decide on the correct coal type.
A dry clay model can also be used, but the water term will include
the clay bound water, not just the free water. It can be removed by
subtracting clay bound water from the tptal to get the free water
answer.
The ash data
points may vary with clay type and other noncombustible mineral
content, so crossplots of lab ash content (by volume) versus log
readings can help pin down these values.
 CALCULATING COAL PROPERTIES
CALCULATING COAL PROPERTIES
The following equations are found in the
coal assay literature and are based on correlations between core
analysis values and log data. Parameters can be tuned by making
your own crossplots. Standard 3- and 4- mineral models using
simultaneous equations, DENSma-Uma crossplots, or Mlith-Nlith
crossplots (or equivalent math) are probably more practical when
the core data correlations are not available.
Initial results are in volume fractions
and are converted to weight fractions by using the density of
each component.
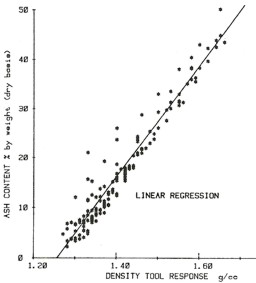 Ash Content: Ash Content:
2: Vash = (DENS - DENSMAcoal) / (2.5 –
DENSMAcoal)
OR 2a: Vash = 0.65 * (DENS - 1.00)
Equations specific to a project area can be obtained by
plotting coal assay data versus density log data, as shown in the
examples at the right.
Fixed Carbon (dry coal):
3: Vfcarb = 0.512 * (1.0 – Vash)
Moisture (free water):
4: Vwtr =
0.461 – Vash
Volatile Matter:
5: Vvolatile = 1.0 – Vash - Vfcarb – Vwtr
All proximate analysis results are
reported in weight fraction or percent. To convert log analysis
volume fractions to weight fractions, use the following:
6: WTash = Vash * DENSash
7: WTfcarb = Vfcarb * DENSfcarb
8: WTwtr = Vwtr * DENSwtr
9 : WTvolatile = Vvolatile * DENSvolatile
10: WTcoal = WTash + WTfcarb + WTwtr + WTvolatile
Mass fractions are as follows
(multiply by 100 to get weight percent):
11:
Wash = WTash / WTcoal
12: Wfcarb = WTfcarb / WTcoal
13: Wwtr = WTwtr / WTcoal
14: Wvolatile = WTvolatile / WTcoal
Weight percent is often used in reports:
15: WT%ash = 100 * Wash
16: WT%fcarb = 100 * WTfcarb
17: WT%wtr = 100 * WTwtr
18: WT%volatile = 100 * WTvolatile
Where:
DENS = density log reading in a coal (g/cc)
DENSMAcoal = matrix density of a coal (g/cc)
DENSxxx = density of a component (g/cc)
Vxxx = volume fraction of a component (fractional)
WTxxx = weight of a component (grams)
Wxxx = mass fraction of a component (fractional)
WT%xxx = weight percent of a component (percent)
 META/LOG
"COAL"
SPREADSHEET -- Coal ASSAY FROM LOG OR CORE ANALYSIS META/LOG
"COAL"
SPREADSHEET -- Coal ASSAY FROM LOG OR CORE ANALYSIS
This
spreadsheet calculates a Coal Assay that can be used to evaluate
coal quality and provides a comparison between core and log analysis
data. It is the same asssay method used to get started with a coal
bed methane analysis.
SPR-14 META/LOG COAL BED METHANE (CBM) CALCULATOR
Calculate coal assay, coal bed methane, adsorbed gas In
place
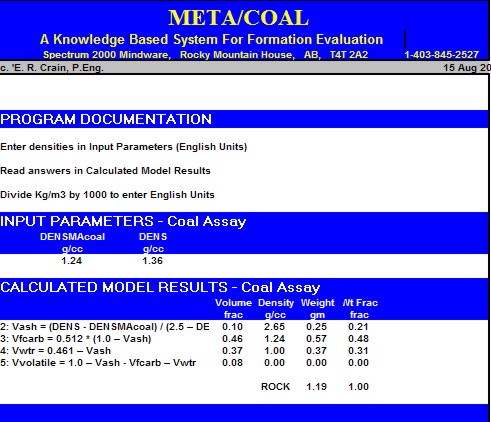
Sample output from "META/COAL" spreadsheet for coal
qusality analysis.
 Coal LOG analysis EXAMPLE Coal LOG analysis EXAMPLE
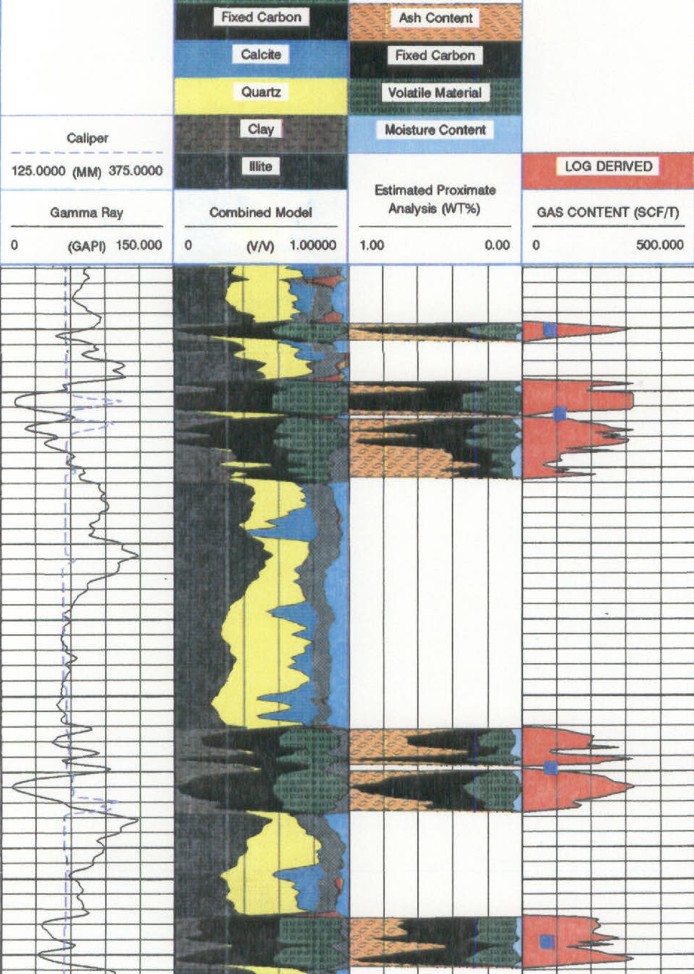
Log analysis of
an Alberta Foothills coal using a model for coal composition (fixed
carbon, volatiles, moisture, and ash (2nd track from the
right). These results can be ca;ibrated to the proximate analysis
from lab measurements.
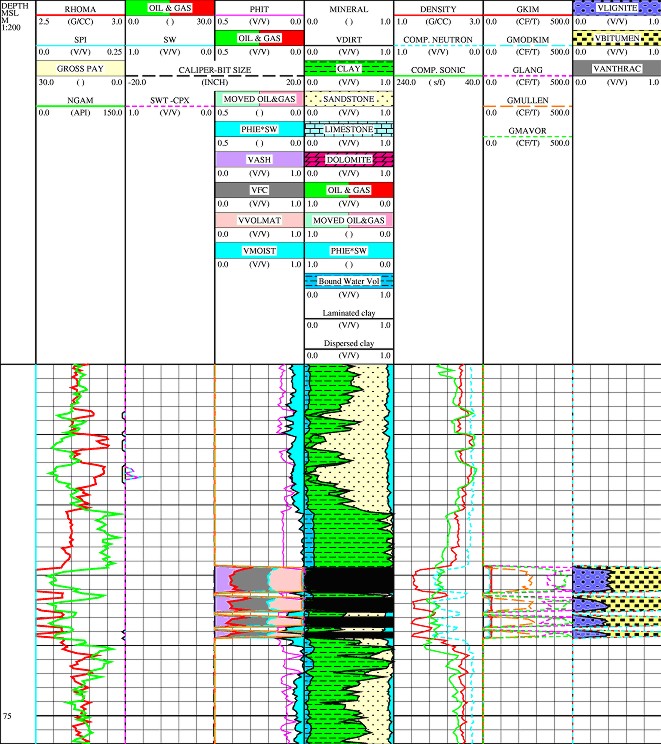
Example of coal
log analysis results using a 3-mineral model for coal type (lignite,
bituminous, anthracite) in right hand track. Zones outside the coal
are analyzed with conventional oil and gas models.
|











 Ash Content:
Ash Content:

